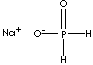| SODIUM
HYPOPHOSPHITE
|
||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 7681-53-0 |
|
| EINECS NO. | 231-669-9 | |
| FORMULA | NaH2PO2.H2O | |
| MOL WT. | 105.99 | |
|
H.S. CODE |
2835.10 | |
| TOXICITY | Oral rat LD50: 1584 mg/kg | |
| SYNONYMS | Phosphinic acid, sodium salt; sodium monophosphate; | |
| Hypophosphorous Acid Monosodium Salt; Natriumhypophosphit (German); Phosphinic Acid Monosodium Salt; Sodium Phosphinate; Fosfinato de sodio (Spanish); Phosphinate de sodium (French); | ||
| SMILES | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | White Crystalline, hydroscopic | |
| MELTING POINT |
|
|
| BOILING POINT | 200 C (Decomposes) | |
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | soluble | |
| pH | 5.5 - 8.5 | |
| VAPOR DENSITY | ||
|
AUTOIGNITION |
|
|
|
NFPA RATINGS |
Health: 1 Flammability: 1 Reactivity: 2 | |
|
REFRACTIVE INDEX |
|
|
| FLASH POINT | Not considered to be a fire hazard but forms spontaneously flammable phosphine gas when heated. | |
| STABILITY | Stable under ordinary conditions but explosive in contact with oxidizers. | |
|
APPLICATIONS |
||
Hypophosphorous
acid is the phosphorus oxo acid or hydroxy phosphine oxide,
with monobasic character. The term phosphinic acid is
for the descriptive presentation of derivatives or salts
which hydrogen atoms bound to
phosphorus are replaced by organic groups, called phosphinates. The term hypophosphite
is also used for any salt, ester or anion derived
from the name of hypophosphorous acid.
Hypophosphorous
acid is a low-melting point compound soluble in water.
It is a powerful reducing agent. Hypophosphorous
acid and its sodium salt (sodium hypophosphite)
are used as powerful reducing agents
in various field. (Hypophosphorous acid has an advantage of easy
implementation in solution.) They are used in:
|
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
White Crystalline | |
|
NaH2PO2.H2O |
100%
min
|
|
|
Na2HPO3 |
0.5% max | |
| SO4 | 0.03% max | |
|
Fe |
0.0003% max | |
|
Ca |
0.005% max | |
|
Cl |
0.03% max | |
| TRANSPORTATION | ||
| PACKING | 25kgs, 50kgs , 500kgs in bag | |
| HAZARD CLASS | Not regulated | |
| UN NO. | ||
| GENERAL DESCRIPTION OF PHOSPHOROUS ACID |
||
|
Phosphorous Acid is a clear to yellowish crystalline solid with a garlic like odour melting at 73 C, decomposes at 200 C. It is very soluble in water and in alcohol. This compound contains one direct P-H bond (which is not very acidic) and only two hydrogens bonded to oxygen (which are acidic). The structure of this material is more correctly written (HO)2HPO. For this reason, this dibasic acid forms two series of salts; the one contains the dihydrogen phosphite ion, H2PO3 - , and the other contains the hydrogen phosphite ion, HPO32-. This is an unstable compound that readily converts to phosphoric acid (H3PO4) in the presence of oxygen or on heating above 180 C. It is prepared by hydrolysis of phosphorus trichloride or tetraphosphorus hexaoxide. Phosphorous acid and its salts called phosphites are used as reducing agents in chemical industry because of easy oxidation property to phosphoric acid. |
||